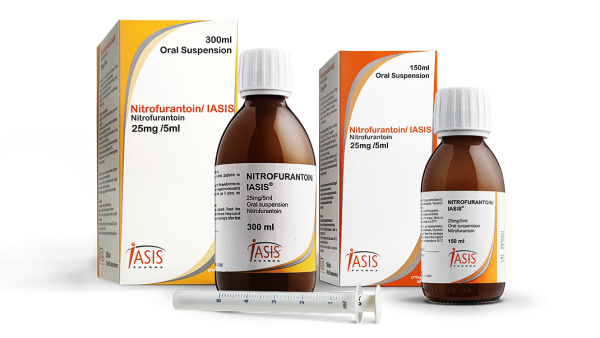
IASIS PHARMA is pleased to announce the Nitrofurantoin/IASIS oral susp. launching in the oral suspension formulation.
This Innovative Pharmaceutical Form is a global market leader. This dosage form is Research and Development by IASIS PHARMA HELLAS S.A.
Each 5ml of oral suspension Nitrofurantoin/IASIS oral susp. contains 25mg of Nitrofurantoin monohydrate which, according to international literature, is the 1st Choice in the Global Guidelines for the Prevention & Treatment of Uncomplicated UTIs.
Nitrofurantoin/IASIS oral susp. is available in the market in the two pack sizes of 150ml & 300ml respectively, indicated mainly for the treatment & prophylaxis against acute or recurrent, uncomplicated lower urinary tract infections or pyelitis, either spontaneous or after surgical operations. It is specifically indicated for the treatment of infections caused by resistant strains of Escherichia coli, Enterococci, Staphylococci, Citrobacter, Klebsiella and Enterobacter.
Nitrofurantoin/IASIS oral susp. is indicated for use in adults and children over three months of age.
It is promoted by PharmaQ, a company of IASIS PHARMA HELLAS S.A.